RNA therapeutic insights by Abzu.
The future of RNA therapeutics is with Abzu.
Delving into RNA insights: The Abzu perspective.
Exploring the RNA insights with Abzu.
Step into the world of RNA therapeutics with Abzu, where we bridge cutting-edge research with practical solutions. Here, we emphasize the groundbreaking potential of RNA insights to transform treatments and therapies, offering a comprehensive look at the future of medicine through the Abzu lense.
Select your favorite media.
Or simply browse all RNA therapeutic insights with Abzu.
Abzu had the pleasure of presenting at Therapeutic Oligonucleotides 2025, taking place in Gothenburg May 14th-15th, 2025.
We shared how we incorporate off-target predictions and physicochemical properties for in silico drug design using our novel explainable AI.
Abzu had the pleasure of presenting at RNA Leaders Europe, taking place in Basel March 3rd-6th, 2025.
At the event, we shared our latest advancements in off-target predictions for in silico drug design using our novel explainable AI.
Explainable AI has huge potential to accelerate disease understanding and drug design by revealing the biological mechanisms that drive drug activity, stability, safety, and delivery.
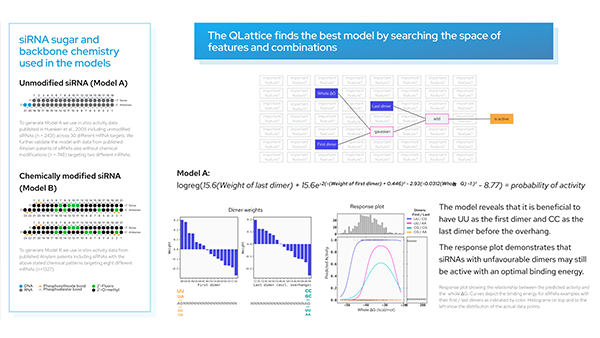
Here we use the QLattice® to generate siRNA activity models from publicly available data to create insights that can be used to design active siRNAs.
Our proprietary technology and brilliant Abzoids ensure that each step that we help our partners take in their pipeline is actually a leap towards viable, in vivo success.
RNA Leaders Europe is the #1 event focused on the development of mRNA, RNAi, ASOs, oligonucleotides, vaccines, microRNAs, genome editing, wider nucleic acids & RNA targets.
A networking event about chartering the future for oligo innovation.
An infinite number of lead candidates does not mean an infinite number of drugs, let alone good drugs.
Lykke Pedersen, Chief Pharma Officer at Abzu, considers the benefits of explainable AI and its applications within the pharma industry.
Oligonucleotides Therapeutics Society (OTS) 2023 poster and keynote.
OTS is a forum to foster academic and industry-based research and development of oligonucleotide therapeutics.
A strategic partnership with Contera Pharma included ASO and siRNA therapeutic designs and target identification.
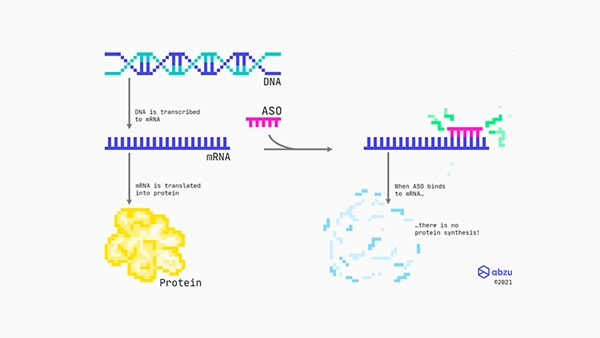
The application designs RNA therapeutics through parallel hypotheses testing of drug targets and individual antisense oligonucleotide (ASO) drug properties.
Our models, interfaced through an application, allowed scientists to modify LNP properties and observe the direct impact.
Investigating the next generation of genetic medicine through RNA-based therapies.
OTS is a forum to foster academic and industry-based research and development of oligonucleotide therapeutics.
Good clinical practice requires responsible conduct and considerations of safety and ethics.
Sharing the latest trends, innovations, and best practices for digitizing R&D assets to accelerate modern science.
Abzu's in-house activity models for RNA therapeutics are best-in-class for designing safe ASOs.
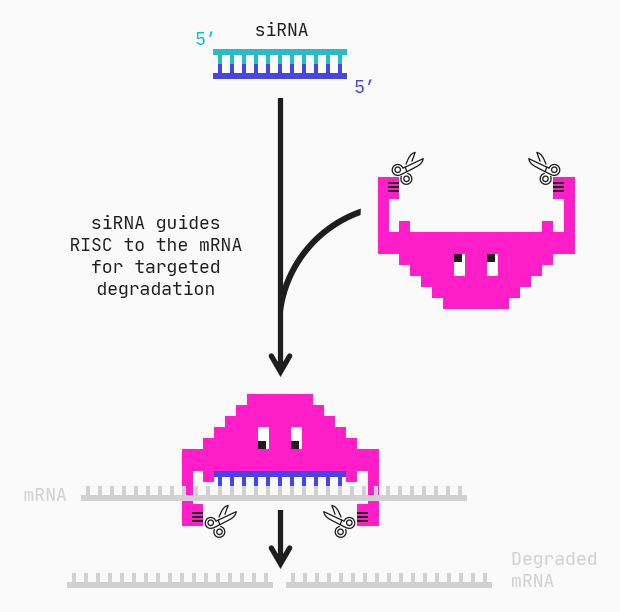
Let’s apply our repositioned selves and the QLattice in a very hot area of research: nucleic acid-based medicines. One particular type, RNA interference (RNAi), involves a process by which specific mRNAs are targeted and degraded.
The increasing application of black-box models gives rise to a range of both ethical and scientific questions.
The two companies aim to have a rapid target screening application available this year, enabling Contera Pharma to advance better nucleic acid-based therapeutics.
Abzu researches into the best way to design antisense oligonucleotides (ASOs), including what features contribute to toxicity.
Leading in short oligonucleotide innovation and design.
Precision therapeutics with ASOs, siRNAs, and antimiRs.
We’re redefining the potential of RNA therapeutics through our specialized workflow with ASOs, siRNAs, and antimiRs.
This focus ensures our therapeutics have high activity and exhibit minimal off-target effects, heralding a new era of precision medicine.
Abzu is your partner in RNA therapeutics design.
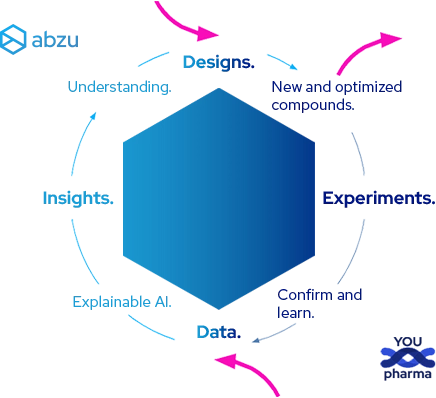
A cycle of innovation and optimization — to RNA therapeutics realization.
Whether we start with designs or your data, Abzu provides you the insights and confidence to accelerate your R&D and transform concepts into market-ready drugs.
Subscribe for
notifications from Abzu.
You can opt out at any time. We’re cookieless, and our privacy policy is actually easy to read.